 » Barrier-free View
» Barrier-free View
| ||||||||
 | ||||||||
 |
 |
 |
| Picture of the 1st Network Meeting of the Herz-Kreislauf-NetzDownloadsArchivExternal job opportunities |
 |
Genomic predictors of heart failure following anthracycline cancer therapy
 |
Subproject leader
Prof. Dr. Leszek Wojnowski
Department of Pharmacology
University of Mainz
Obere Zahlbacher Str. 67
55101 Mainz
email: wojnowski@uni-mainz.de
Phone: 06131-3933 460
Anthracyclines, e.g doxorubicin or daunorubicin, are antineoplastic agents successfully used in therapies of various solid and haemotopoietic malignancies. A major limitation of anthracyclines is the cardiotoxicity. Different types of anthracycline-induced cardiotoxicity have been defined: Acute cardiotoxicity occurs during treatment, mostly in the form of arrhythmias, and it is usually manageable. Chronic toxicity manifests as congestive heart failure months, years or even decades after the treatment. The causal relationship between acute and chronic form is unclear. The responsible mechanisms remain a matter of debate, but the prevailing hypothesis postulates anthracycline-induced generation of reactive oxygen species (ROS) leading to damage of cardiomyocytes.
Cardiotoxicity is generally dose-dependent. This has led to dose limitation, e.g. the total dose of doxorubicin should not exceed 550 mg/m2 body surface since higher doses dramatically increase the risk of cardiac damage. However, dose restriction may adversely affect the therapeutical outcome of anthracyclines. Importantly, interindividual differences in the susceptibility to cardiac damage are well documented. Some patients developed heart failure after 100 mg/m2 body surface, whereas others showed no adverse reactions after accidental overdosing with 2 g/m2 body surface. This suggests that sensitivity to anthracyclines may be affected by the individual genetic makeup. This hypothesis is supported by several studies with transgenic and knockout mice, which demonstrated altered sensitivity to anthracyclines. For example, the overexpression of MDR1 (multiple drug resistance 1) led to a protection of the heart against doxorubicin. However, the relevance of these findings to human cardiotoxicity is unknown.
The aim of our study is to assess the impact of the individual genetic makeup on doxorubicin-induced cardiotoxicity. Identification of the involved genes and their variants could help to develop safer individualized therapies and also give further insight into the pathophysiology of this and other types of heart failure.
We looked for genetic markers predictive for doxorubicin-induced heart failure in 1400 patients with Non-Hodgkin lymphoma. These patients were enrolled in the prospective, multicenter randomized NHL-B trial conducted by the German High Grade non Hodgkin’s Lymphoma Study Group (DSHNHL) between 1993 and 2000. 6.4% of patients developed cardiotoxicity. Genomic DNA samples from these patients as well as from unaffected patients from the same study were subjected to genotyping. The genes and polymorphisms screened were derived from genes encoding proteins involved in anthracycline transport and metabolism, in ROS metabolism, DNA repair, endothelial physiology, muscle structure and contraction, inflammation, cell cycle, or generally predisposing to heart failure. We identified several associations, three of which in subunits of the NAD(P)H oxidase, which is a key enzyme in the cellular generation of superoxide. The first one is the His72Tyr variant of the CYBA gene-encoded subunit p22phox, and the second a T/A-SNP in the second intron of the RAC2 gene, a NAD(P)H oxidase activator. Additionally, there was a significant association with a variant of NCF4, a subunit responsible for downregulation of the NAD(P)H oxidase. Two further genetic associations were found in genes encoding the efflux transporters MRP1 (multi drug resistance protein 1; Gly671Val) and MRP2 (the Val1188Glu-Cys1515Tyr haplotype) (1). Both MRP proteins are known to transport doxorubicin and at least MRP1 is expressed in cardiomyocytes.
The identified associations are being verified by appropriate expression techniques, in genetically modified mouse models, and by epidemiological means. The MRP1 variant Gly671Val and the MRP2 haplotype variant Val1188Glu-Cys1515Tyr are expressed in Xenopus laevis oocytes and their functional effects characterized in transport studies. To this end, full-length cDNAs of MRP1 and MRP2 have been cloned in-frame with the fluorescent protein EGFP into a suitable expression vector. The desired MRP1 and MRP2 point mutations were introduced by site-directed mutagenesis and confirmed by sequencing. Vector-derived cRNAs were then injected into oocytes and analyzed after 3 days of incubation for protein expression and transport. Figure 1 shows fluorescent micrographs of cryostat sections from oocytes injected with MRP1-EGFP and MRP2-EGFP cRNA, demonstrating correct localization of each fusion protein in the plasma membrane
 |
Figure 1: Fluorescent micrographs of cryostat sections from Xenopus laevis oocytes injected with MRP1-EGFP (left panel) and MRP2-EGFP (right panel) fusion cRNA. Note the strong expression of either fusion protein in the plasma membrane |
An anti-EGFP-antibody detected bands of the expected size in western blot of lysed oocytes (not shown). To determine the transport ability of the fusion proteins, oocytes were incubated for 6 h in a solution containing 40µM radioactively (14C) labelled doxorubicin. The accumulated radioactivity was assayed by liquid scintillation spectroscopy of lysed oocytes. As shown in figure 2, the accumulation of doxorubicin was decreased in oocytes injected with RNA encoding MRP proteins, as compared with oocytes injected with water. This result is consistent with the functionality of either fusion protein as an efflux doxorubicin transporter in oocytes. Characterization of the transport kinetics and comparisons between wild-type and mutant proteins are in progress.
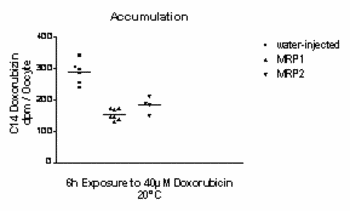 |
Figure 2: Accumulation of doxorubicin is decreased in oocytes injected with RNA encoding hMRP protein. |
The significance of associations between polymorphisms in the NAD(P)H oxidase subunits and individual sensitivity to doxorubicin are verified in mice deficient in this enzyme. The NAD(P)H oxidase deficiency has been achieved by disruption of the gp91 gene, which encodes the nox2 subunit of the enzyme. Heart failure is induced by weekly injections of doxorubicin over a period of 5 weeks. Heart function is assessed by echocardiography and compared to similarly treated wild-type mice. Within 10 weeks after the treatment onset wild-type mice developed a congestive heart failure characterized by dilatation and reduced contractility of the left ventricle (Fig. 3). In contrast, the gp91 knockout mice did not show any significant impairment of heart function. This observation supports the true character of the associations between NAD(P)H oxidase polymorphisms and doxorubicin-induced heart failure found in humans. Currently we are investigating the biochemical mechanism of the interaction between the NAD(P)H oxidase and doxorubicin.
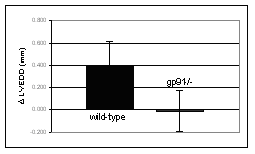 | 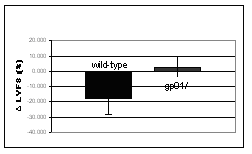 | |
Figure 3: Effect of chronic doxorubicin treatment on left ventricular end-diastolic dimension (LVEDD) and fractional shortening (LVFS) assessed by echocardiography in C57Bl/6 mice deficient in the NAD(P)H oxidase subunit gp91 (n=11) and in wild-type mice of the same background (n=18). Measurements were performed four weeks after completion of the treatment. Data shown as mean ± SD. | ||
In addition, we are looking for other genetic loci related to anthracycline-induced heart failure by means of quantitative trait analysis (QTL). Thus far, we have identified two genetically distant mouse inbred strains, C57Bl/6 and Balb/c, which are differentially sensitive to doxorubicin (not shown). The sensitive strain C57Bl/6 was then crossed with the insensitive Balb/c mice. The resulting F1 C57Bl/6xBalb/c mice were insensitive to doxorubicin, indicating a recessive inheritance of doxorubicin sensitivity in C57Bl/6 mice. Therefore, the F1 mice have been backcrossed to C57Bl/6 mice. The resulting N2 mice are now being analyzed in terms of doxorubicin sensitivity. By typing of 120 informative markers in sensitive and insensitive N2 mice we hope to identify genomic regions responsible for sensitivity of C57Bl/6 mice to doxorubicin. This will be followed by fine-mapping of these regions to identify the responsible gene loci.
The associations between doxorubicin-induced cardiotoxicity and NAD(P)H oxidase or MRP1 and MRP2 gene variants will be further verified by genotyping DNA samples from additional clinical studies. To this end, DNA samples have been collected from 1200 patients treated with doxorubicin for lymphomas within the RICOVER60 study. The phenotypic characterization of these patients is in progress. Since genotyping has recently become possible on a genome-wide basis, we have conducted two projects aimed at the evaluation of the underlying new technologies. Firstly, we have analyzed the fidelity of genome-wide amplification of minute amounts of patient genomic DNA. The results (2) indicate a high fidelity of the amplification, although 7% of the polymorphic loci are lost in the process. Secondly, we investigated the suitability of first-generation SNP arrays to conduct genome-wide association studies. Depending on the allelic frequency, allelic penetrance, linkage disequilibrium, and the statistical procedures applied, the technique allows for the identification of up to 25% of the simulated disease loci (3). A similar evaluation of a second-generation array is in progress.
The elucidation of the genetic basis of anthracycline-induced cardiotoxicity will be continued using the mutually complementary resources of clinical studies, mouse genetics, and molecular expression systems. Specifically, we will continue confirming the functional relevance of the identified associations between this disorder and MRP and NAD(P)H oxidase gene variants. Additional candidate genes will be identified in the ongoing QTL analysis and verified experimentally. Further candidate genes could be identified using a genome-wide association approach, which has recently become feasible.
Publications:
1. Burk O, Koch I, Raucy J, Hustert E, Eichelbaun M, Bröckmöller J, Zanger UM, Wojnowski L: The induction of CYP3A5 in human liver and intestine is mediated by the xenobiotic sensors PXR and CAR. J Biol Chem, 279:38379-85, 2004
2. Wojnowski L et al. NAD(P)H oxidase and MRP genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Arch Pharmacol. 2004, 369(s1): R150.
3. Wojnowski L, Brockmöller J: Single nucleotide polymorphism characterization by mRNA expression imbalance assessment. Pharmacogenetics 14:267-9, 2004
4. Tzvetkov MV, Becker Ch, Kulle B, Nürnberg P, Brockmöller J, Wojnowski L: Genome-wide single-nucleotide polymorphism arrays demonstrate high fidelity of multiple displacement-based whole-genome amplification. Electrophoresis 26: 710-715, 2005
5. Kulle B, Schirmer M, Toliat MR, Suk A, Becker Ch, Tzvetkov MV, Brockmöller J, Bickeböller H, Hasenfuß G, Nürnberg P, Wojnowski L: Application of genome-wide SNP arrays for detection of simulated susceptibility loci. Human Mutation, in press
more information around the working group: