 » Barrier-free View
» Barrier-free View
| ||||||||
 | ||||||||
 |
 |
 |
| Picture of the 1st Network Meeting of the Herz-Kreislauf-NetzDownloadsArchivExternal job opportunities |
 |
Von Willebrand Factor
 |
Subproject leader
Prof. Dr. Reinhard Schneppenheim
Clinic of Pädiatric Hämatology und Oncology
University of Hamburg-Eppendorf
Martinistr. 52
20256 Hamburg
email: schneppenheim@uke.uni-hamburg.de
Phone: 040-42803 4270
Von Willebrand-factor (VWF) plays a key role in primary haemostasis and is implicated in haemorrhagic as well as in thromboembolic disorders. Quantitative or functional deficiencies cause von Willebrand disease (VWD), characterised by mucocutaneous bleeding or - in the severe form - additionally by haemophilia-like symptoms such as joint bleeding and muscle bleeding. VWF functional activity in primary haemostasis is tightly regulated by a specific metalloprotease, recently identified as ADAMTS13. Acquired or inherited deficiency of ADAMTS13 cause thrombotic-thrombocytopenic purpura (TTP), a life-threatening microangiopathic disorder on the basis of unproteolysed hyperactive ultralarge VWF multimers. This dual role of VWF in two particular diseases is an attractive feature for studies on the interaction of VWF and ADAMTS13 mutants and polymorphisms, respectively, in bleeding and thrombotic disorders. Indeed it has been shown that elevated levels of VWF correlate with a higher risk of cardiovascular events like myocardial infarction. The basis of elevated VWF levels, however is not clear. Since VWF is considered an acute phase protein its levels will rise during episodes of inflammation. Polymorphisms in the promotor region of VWF have been studied, however, no clear correlation was found between these markers and cardiovascular events. The VWF gene is highly polymorphic. Many SNPs have been identified, several of them resulting in amino acid substitutions which could theoretically influence VWF function. ADAMTS13 also displays a high degree of genetic heterogeneity, as 17 common polymorphisms have been described to date, some of them resulting in amino acid substitutions.
The interplay of VWF synthesis, constitutive and stimulated secretion, the relation of high and smaller molecular weight VWF multimers (which in turn depends on the action of ADAMTS13) and VWF clearance from the circulation results in the actual level of VWF and – at particular occasions -
independently in its functional activity. Therefore VWF and ADAMTS13 in concert will contribute to the risk of arterial thrombotic events on the background of other risk factors of cardiovascular diseases. VWF together with platelets may then be the final effector of occlusion.
This mechanism suggests that functional polymorphisms in these genes, either separately or interacting could modify the particular risk derived from this system.
Whereas the level of VWF can be measured exactly by the VWF:Ag, its functional activity is described by several less accurate assays including Ristocetin Cofactor (VWF:RCo), collagen binding (VWF:CB) and factor VIII binding (VWF:FVIIIB).
1) To study the impact of the level of VWF alone versus its functional activity on triggering cardiovascular events in a cohort of female patients and controls from the CORA study, including the cases of 210 consecutive women with incident coronary heart disease and 230 population-based randomized women from the same neighborhood as controls by determination of VWF:Ag and VWF:CB.
2) To catalogue a set of 34 single nucleotide polymorphisms (SNP) of the VWF gene in order to establish the common VWF haploytpes present in our population.
3) To catalogue a set of 17 SNP of ADAMTS13 in order to establish the common ADAMTS13 haploytpes present in our population.
4) To estimate the impact of particular VWF and ADAMTS13 haplotypes alone and in combination on the risk of cardiovascular events.
1) VWF:Ag and VWF:CB
179 patients and 229 controls were evaluable for our study. We found a significant correlation between both VWF:Ag and VWF:CB and the risk of incident coronary heart disease in patients compared to the control group (Fig. 1). However, the ratio between VWF:CB and VWF:Ag was close to 1 suggesting that the VWF functional activity was not independent from VWF:Ag and that VWF:Ag elevation itself correlates with risk.
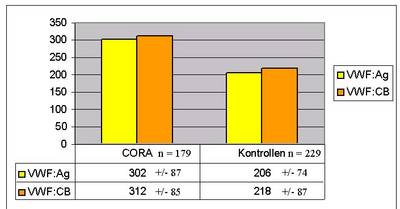 |
Fig 1: VWF Ag and VWF:CB in cases and controls from the Cora study. |
2) VWF haplotypes
To date 34 SNP were analyzed in 88 individuals and 8 families with 24 members. The data will now be evaluated by haplotype analysis using the Expectation-Maximization (EM) Algorithm by the computer program FAMHAP.
3) ADAMTS13 haplotypes
Haplotypes from 31 individuals, 7 trios, 1 nuclear family with two children and 1 nuclear family with four children, were established by applying the Expectation-Maximization (EM) Algorithm by the computer program FAMHAP.
We identified 4 major haplotypes with frequencies above 0.05 and 2 additional haplotypes with frequencies > 0.03. These 6 haplotypes make up 75 % of 20 haplotypes in total, derived from our calculations (Fig. 2).
On the basis of established haplotypes of VWF and ADAMTS13 a set of diagnostic SNP will be chosen for further haplotype analysis of a larger cohort. The major haploytypes which include amino acid polymorphisms will be analyzed in cases and controls from the CORA study, both individually and in concert of VWF and ADAMTS13 by using high throughput techniques. If amino acid polymorphisms or specific haplotypes of VWF and/or ADAMTS13 contribute to the risk of myocardial infarction, functional studies of such polymorphisms using recombinant technology will follow.
A correlation between the VWF/ADAMTS13 system and the risk of cardio-vascular events would help to decide for the appropriate prophylaxis against a 2nd event. Such patients could probably profit in particular from platelet aggregation inhibitors.
Publications:
1. Meade TW, Cooper JA, Stirling Y, Howarth DJ, Ruddock V, Miller GJ. Factor VIII, ABO blood group and the incidence of ischaemic heart disease. Br J Haematol. 1994; 88:601-7.
2. Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med. 1995;332:635-41.
3. Morange PE, Simon C, Alessi MC, Luc G, Arveiler D, Ferrieres J, Amouyel P, Evans A, Ducimetiere P, Juhan-Vague I; PRIME Study Group. Endothelial cell markers and the risk of coronary heart disease: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) study. Circulation. 2004;109:1343-8.
4. van der Meer IM, Brouwers GJ, Bulk S, Leebeek FW, van der Kuip DA, Hofman A, Witteman JC, Gomez Garcia EB. Genetic variability of von Willebrand factor and risk of coronary heart disease: the Rotterdam Study. Br J Haematol. 2004; 124:343-7.
5. Zyriax BC, Boeing H, Windler E. Nutrition is a powerful independent risk factor for coronary heart disease in women-The CORA Study: a population-based case-control study. Eur J Clin Nutr. 2005, [Epub ahead of print].
more information around the working group:
http://www.uke.uni-hamburg.de/kliniken/haematologie/index.php?