 » Barrier-free View
» Barrier-free View
| ||||||||
 | ||||||||
 |
 |
 |
| Picture of the 1st Network Meeting of the Herz-Kreislauf-NetzDownloadsArchivExternal job opportunities |
 |
Genomics of diastolic heart failure
 |
Subject leader
Prof. Dr. Gerd Hasenfuß
Heart Center Göttingen
Robert-Koch-Str. 40
37075 Göttingen
email: hasenfus@med.uni-goettingen.de
Phone: 0551-396 351
Diastolic dysfunction (preserved ejection fraction (EF) and abnormal diastolic function) as well as diastolic heart failure (diastolic dysfunction and a clinical syndrome characterized by the symptoms and signs of heart failure) have only recently been recognized as independent diseases [1].
Opposing data have been published regarding the prevalence of this disease, which ranges at least between 14% to 41% [2]. The wide range of prevalence is likely due to differences in diagnostic criteria and different echocardiographic measurements. However, it can be estimated that about 40% of all patients presenting with the symptoms of heart failure have a primary defect in diastolic function. Hypertension, age, obesity and diabetes are the major risk factors for this disease.
In contrast, not much is known about the mechanistic and genetic basis of this disease. Therefore our aim is the identification of genetic markers, predisposing to diastolic dysfunction and heart failure. We shall also analyze signaltransduction pathways leading to the disease (analysis of human myocardial tissue arrays together with the German Cancer Center, DKFZ). Another goal is the development of novel therapeutic strategies to prevent or treat this disease.
One of our goals within the current NGFN project is to develop and to analyze new animal models with defects in diastolic function. Diastolic function is primarily influenced by the extracellular matrix (i.e. fibrosis), by cytoskeletal components (i.e. Z-disc proteins), and / or by defects in calcium cross bridge function.
T-cap is a 19 kDa protein, located at the Z-disc of cross striated muscles. It has been shown to interact with titin [3], with minK, the potassium channel b subunit [4], with calsarcins 1-3 [5] and we have been able to discover its interaction with the muscle LIM protein (MLP) [6]. Interestingly, it has also been demonstrated that mutations in this gene are a cause of limb girdle muscular dystrophy 2 G [7] and our group linked mutations in this gene to human dilated cardiomyopathy (DCM) and implicated t-cap in cardiac mechanosensing [6, 8].
To gain more insight into the underlying molecular mechanisms we engineered within the current NGFN project a t-cap null mouse model by homologous recombination. Using this model, we started already to perform muscle strip experiments, proteome and transcriptome analysis (in cooperation with the Ressource Center RZPD in Heidelberg) as well as to perform a defined analysis of specific signal transduction pathways using protein arrays.
Hemodynamic analysis revealed that this mouse exhibits a severe, isolated diastolic dysfunction, thus making the t-cap deficient mouse an ideal model to study this condition (please see figure 1).
MLP deficient mice are well known to develop a severe form of DCM [9] and it has been shown, that mutations in this gene cause hypertrophic cardiomyopathy (HCM) [10] as well as DCM [6]. We have been able to detect a severe diastolic dysfunction in MLP-/- mice at a very early age and even before the onset of heart failure (4 weeks). We detected also a defect in mechanical stretch sensing, which suggests a link between diastolic dysfunction and mechanosensing [11]. We have also been able to discover a MLP mutation in patients with DCM (W4R MLP). This mutation is highly significantly associated with DCM (p<0.01, LOD score: 2.62) and by haplotype analysis we were able to describe the first founder effect in DCM [6] (please see figure 2).
We have also generated the W4R MLP knock in mouse, a model harboring the above mentioned mutation.
Preliminary data within the current NGFN project on W4R MLP knock in mice point to a defect in diastolic function in these mice.
The t-cap and MLP deficient mice are representative models for Z-disc protein related defects in diastolic function.
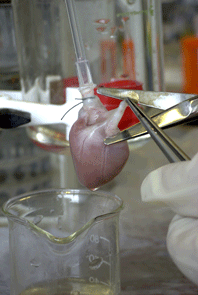 |
Fig 1: Isolated heart in Langendorff perfusion modus. |
Myosin binding protein C (MybPC) is a major component of cardiac sarcomeres. It is important for the structural integrity as well as myocardial function. Mutations in this gene have been shown to be the cause of both, HCM and DCM [12, 13]. Notably, a major feature of HCM in humans is a defect in diastolic function.
However, next to nothing is known about the function of MybPC. Therefore, we generated MybPC knock out mice and analyzed these animals by echocardiography, hemodynamic analysis as well as different biophysical techniques. We found that homozygous MybPC deficient mice develop eccentric hypertrophy with a severe defect in diastolic function, whereas heterozygous MybPC knock out mice develop a phenotype comparable to the phenotype observed in patients with HCM [14].
MybPC interacts with myosin and has the potential to influence the calcium sensitivity of myofilaments. This model is therefore representative for a diastolic dysfunction induced by changes in calcium cross bridge function.
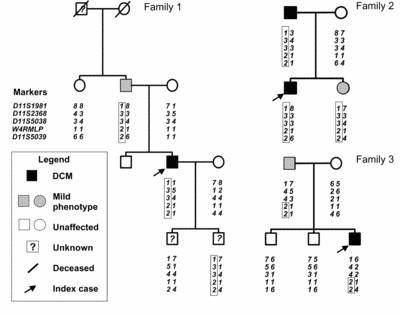 |
Fig. 2 Three pedigrees of patients carrying the W4R MLP |
Since MybPC deficient mice (diastolic dysfunction induced by changes in calcium cross bridge function) develop eccentric hypertrophy and since MLP serves as a mechanosensor with the potential to induce myocardial hypertrophy, we wondered whether the loss of MLP might lead to a normalization of myocardial function in MybPC null mice. To test this hypothesis MLP and MybPC double knock out mice have been generated and we are in the process of analyzing this line within the current NGFN project.
Preliminary echocardiographic data (left ventricular diameters, fractional shortening) point indeed to an improved myocardial function in the double knock out mice in comparison to the corresponding MybPC knock out, MLP heterozygous littermates. Real time PCR revealed a higher sarcoplasmic reticulum ATPase (SERCA) mRNA expression in the double knock out line, which might be able to explain, at least in part, the improved myocardial function in this line. In addition, the double knock out line does not develop myocardial hypertrophy. The available data point to an improvement of function when we ablate MLP in the MybPC deficient background.
Our aim is to identify genetic variants predisposing to the development of diastolic dysfunction in the general population and in patients with risk factors such as hypertension, diabetes, obesity and/or higher age. We shall also identify genes predisposing to the progression from asymptomatic diastolic dysfunction to clinically apparent diastolic and combined diastolic and systolic heart failure.
Core of this analysis is the KORA/MONICA cohort (1650 individuals, in cooperation with the Institute of Epidemiology, GSF, Munich) and our own populations consisting of either 540 patients with risk factors for diastolic dysfunction and 1000 patients with asymptomatic diastolic dysfunction, which will be followed up for the development of diastolic and/or systolic heart failure.
Therefore genes either affecting relaxation (calcium, cross bridge function) and/or myocardial stiffness (cytoskeletal, extracellular matrix) are in the center of our interest. Candidate genes will either be sequenced or single nucleotide polymorphisms will be analyzed (together with the Cologne Center for Genomics (CCG, Köln)).
Proteins affecting diastolic function will also be analyzed by immunohistochemistry using human myocardial tissue arrays (in cooperation with the German Cancer Center DKFZ).
In summary, MLP is a mechanosensor and its loss is associated with a DCM phenotype in mice. In addition, loss of function mutations in this gene can cause cardiomyopathy in humans.
Notably, in the MLP knock out mouse as well as in patients with the W4R MLP mutation, diastolic dysfunction has been observed. Therefore it is possible that MLP mutations cause cardiomyopathy by inducing diastolic dysfunction via a defect in mechanosensing.
The MLP knock out, the t-cap knock out, the MybPC knock out as well as the W4R MLP knock in mice will be used as models to study in depth diastolic dysfunction.
Analysis of pathways underlying diastolic heart failure will provide invaluable tools to screen for patients with this disease and to develop innovative strategies to combat heart failure.
Population studies will link certain SNPs to diastolic dysfunction in humans and, in combination with the data obtained from animal models, will enable us to diagnose and treat affected patients efficiently.
Developmental process from embryonic
shem cells to differentiated, contactile
cardiac myocytes in mouse or rat, respectively.
additional pictures and information ->
 |
Working group Prof. Dr. Hasenfuß |
Publications:
1. Andreas, S., H. Reiter, et al. (2004). "Differential effects of theophylline on sympathetic excitation, hemodynamics, and breathing in congestive heart failure." Circulation 110(15): 2157-62.
2. Damy, T., P. Ratajczak, et al. (2004). "Increased neuronal nitric oxide synthase-derived NO production in the failing human heart." Lancet 363(9418): 1365-7.
3. Hasenfuss, G., L. S. Maier, et al. (2002). "Influence of pyruvate on contractile performance and Ca(2+) cycling in isolated failing human myocardium." Circulation 105(2): 194-9.
4. Hasenfuss, G. and W. Schillinger (2004). "Is modulation of sodium-calcium exchange a therapeutic option in heart failure?" Circ Res 95(3): 225-7.
5. Hasenfuss, G. and T. Seidler (2003). "Treatment of heart failure through stabilization of the cardiac ryanodine receptor." Circulation 107(3): 378-80.
6. Hermann, H. P., B. Pieske, et al. (1999). "Haemodynamic effects of intracoronary pyruvate in patients with congestive heart failure: an open study." Lancet 353(9161): 1321-3.
7. Knöll, R., M. Hoshijima, et al. (2002). "The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy." Cell 111(7): 943-55.
8. Kogler, H., O. Hartmann, et al. (2003). "Mechanical load-dependent regulation of gene expression in monocrotaline- induced right ventricular hypertrophy in the rat." Circ Res 93(3): 230-7.
9. Pieske, B., L. S. Maier, et al. (2002). "Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium." Circulation 106(4): 447-53.
10. Teucher, N., J. Prestle, et al. (2004). "Excessive Sarcoplasmic/Endoplasmic Reticulum Ca2+-ATPase Expression Causes Increased Sarcoplasmic Reticulum Ca2+ Uptake but Decreases Myocyte Shortening." Circulation.
more information around the working group:
http://www.herzzentrum.med.uni-goettingen.de